
(Courtesy of CMD) Last month, Dr. Daniel Popowitz, board-certified foot and ankle surgeon and chief of foot and ankle at CMD in New Jersey, performed the first-ever-in-New Jersey hammertoe correction, using the new innovative OSSIOfiberTM implant at Hudson Regional Hospital.
OSSIO, Inc., an orthopedic fixation company founded in Israel, is committed to transforming the orthopedic experience for patients, physicians and payers. Founded in 2014 by Orahn Preiss-Bloom, a New York native and alumnus of TABC, its vision is to provide the first credible replacement to metal implants in the multi-billion-dollar global orthopedic fixation market with its OSSIOfiberTM Intelligent Bone Regeneration Technology.
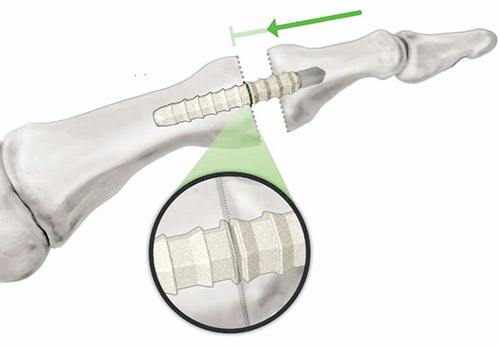
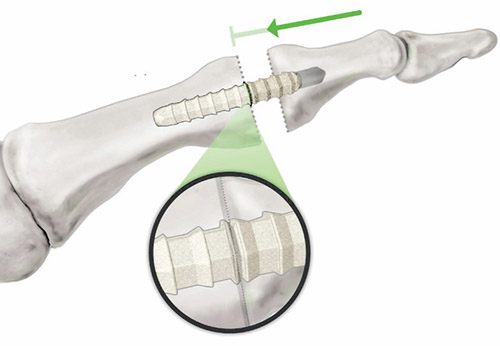
The OSSIOfiberTM Bone Pin Family received market clearance from the U.S. Food and Drug Administration (FDA) in January and the initial product offering is applicable for use in the foot and ankle segment for the treatment of forefoot conditions where hardware removal surgeries are prevalent. Offering a new category of non-permanent fixation material, the OSSIOfiberTM Intelligent Bone Regeneration Technology features a first-of-its-kind, proprietary bio-integrative material that provides stability and secure bone fixation during the healing process and gradual integration into the native anatomy, ultimately leaving no permanent hardware behind. Combining unparalleled mechanical strength and natural bone healing in a non-permanent implant, OSSIOfiberTM is designed to fully incorporate into the native anatomy without any adverse biologic response.
“I am very excited to be using this new technology. OSSIOfiberTM shows real promise to become the first implant to replace metal pins and screws,” said Dr. Popowitz. “I am honored to have performed the first case in New Jersey and I look forward to adding these bio-integrative implants and materials into my surgical practice. The company, based in Caesarea, Israel, is already moving ahead with production of screws, anchors and plates. “Perhaps metal implants will soon be a thing of the past.”
Just last week, OSSIO, Inc. announced that it was the recipient of the 2019 Frost & Sullivan North America New Product Innovation Award. The company received the prestigious award within the market of bone regeneration fixation devices, having demonstrated best-in-class qualities in unique areas including understanding demand, nurturing the brand and differentiating from the competition.
“The U.S. market anticipation for OSSIOfiberTM has been overwhelmingly positive to date,” said Brian Verrier, CEO, OSSIO. “We are poised to initiate broader U.S. commercialization of the implant system in the coming weeks, offering surgeons and their patients a new standard of care in orthopedic fixation.”
Designed for rapid bone in-growth, regeneration and replacement, OSSIOfiber™ Intelligent Bone Regeneration Technology is a first-of-its-kind implant material stronger than cortical bone that leaves nothing permanent behind. OSSIOfiberTM is engineered to provide the strength required for functional fixation and allows for full integration into the native anatomy without adverse biological response.
The proprietary OSSIOfiberTM technology can address many surgical applications through the manufacturing of endless implant designs, including pins, screws and plates. The company intends to pursue multiple applications in the distal extremity, trauma, sports, reconstruction, pediatrics and spine segments.
If you suffer from hammertoe deformities, call 201.-510-3777 to learn more about this new innovative technology and how it can benefit you.