
Research Associate at Southern Cross University
Most of what we taste we actually smell. The only sensations that we pick up in our mouth are sweet, sour, bitter, umami and salty. Without its smell, coffee would have only a sour or bitter taste due to the organic acids. Try it with your next cup of coffee–hold your nose as you take your first sip.
The rich satisfying sensation of coffee is almost entirely due to the volatile compounds produced when we roast coffee beans.
The compounds that are formed in the roasting process are very similar to any other compound that is formed in the cooking process. The smell of baking bread is from compounds produced when a sugar reacts with a protein in what is called a Maillard reaction.
Not every scent is as welcoming as freshly baked bread, though. Our sense of smell has developed over millennia to detect dangerous compounds.
Cadaverine and putracine, produced in rotting meat, can be detected by our nose at very low concentrations. The same can be said of sulphur-containing compounds such as hydrogen sulphide–rotten egg gas–which is detected by our nose at levels of parts per billion.
The upshot of this is that we do not detect all compounds in our surroundings to the same extent. For example, to us water is completely odorless although it may be very concentrated in the atmosphere.
Odour chemists have developed a system called odor activity values which show how we respond to particular compounds. This has an influence on how we experience a complex mixture of stimuli.
Flavorists and perfumists have developed a series of descriptors, or words that are used to describe a particular smell. Using gas chromatography equipped with a sniffer port, chemists are able to smell individual compounds as they come off the gas chromatography column and apply a description to what they experience.
Words such as fruity, earthy, flowery, caramel-like, spicy and meaty are used to describe the odour of individual compounds. It is this complex mixture of volatile organic compounds that we can identify with a particular food. The smell of baking bread can easily be distinguished from the smell of cooking cabbage; a lamb roast from a pork roast.
Yet it is not one compound that is responsible for the odour that we experience, but a complex mixture of hundreds of different compounds.
What we smell in coffee
Approximately 800 different compounds are produced in the coffee-roasting process. These thermal degradation reactions decompose sugars and proteins to form the volatile compounds that we smell.
Most of these reactions take place within the thick walls of coffee bean cells, which act as tiny pressure chambers. Not all of these 800 compounds cause the same response in the olfactory membrane in your nose, though.
Green (unroasted) coffee tastes very grassy when brewed. You still get the organic acids and caffeine in the brew but it lacks the full sensation because there are few volatile compounds due to the lack of roasting.
The profile of roast coffee includes only 20 major compounds, but it is the influences of some of the minor compounds that determine the overall taste that we experience.
When chemists are analyzing the volatile compounds in coffee a huge range of different odor qualities are experienced.
Some of the nitrogen-containing compounds such as pyridine can actually smell quite foul, while others can smell quite fruity.
Other compounds have descriptors such as putrid or rancid. One compound, 5- methyl furfural, is described only as coffee-like. But it is the rich mixture of hundreds of different volatile compounds that, when we smell it, can only be described as “coffee.”
The Perfect Cup of Coffee
It’s hard to get a bad coffee these days. Plenty of baristas have fine-tuned the process of making espresso, but really there are only a handful of variables they can control:
1. coarseness of the grind
2. temperature of the extraction
3. extraction time
4. the all-important coffee-to-water ratio.
Coffee roasters and barista schools have produced many impressively complex charts plotting grams of coffee against volume of water overlaid with concentration and yield. In the middle is the ideal weight/ volume/ concentration yield target for the perfect cup of coffee.
(Of course, if you prefer a latte, cappuccino or flat white, the milk is a whole other story.)
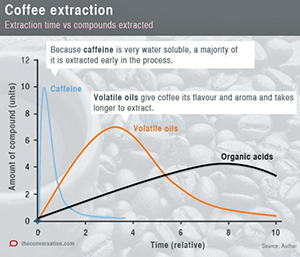
Here is a little graphic of my own that I will use to describe what happens when we change our four variables.
On the horizontal axis we have relative time, on the vertical axis the numbers represent amount. The curves are extraction profiles.
Caffeine is very water soluble and the vast majority of the caffeine is extracted early. The volatile oils, which give coffee its complex flavor and aroma, extract more slowly. The organic acids, which make coffee taste bitter, are extracted the most slowly of all.
So let’s go through each of our four variables in turn.
1. Grind
The coarseness of the grind and the extraction time are inextricably linked. The finer a coffee is ground, the more surface area there is. Conversely, the larger the grind, the smaller the surface area.
Let’s consider the two ends of the extreme. If we grind coffee as fine as talcum powder we have maximized the surface area available for extraction. Therefore, we can very quickly extract the target compounds – but perhaps too quickly for some people’s tastes.
Turkish coffee is very finely ground and boiled. This produces a very strong and bitter coffee and because of the fineness of the grind often contains a lot of suspended solids (muddy). The finely ground material may block filters too, causing the extraction to go on for too long–or not allow the water to pass through at all.
At the other end of the spectrum, let’s consider whole coffee beans. Of course, given enough time, we can extract unground coffee. This is quite wasteful of the coffee beans because the hot water may not penetrate all the way to the interior of the bean, so we throw away unextracted material.
Obviously, the optimum grind (coarseness) is somewhere between these two extremes, where we match the residence time of the hot water (flow rate) across the ground coffee beans with our ideal caffeine/ volatile oil/ organic acid ratio.
If you get a cup of coffee produced from a quality bean but it is too weak and insipid, the coffee may have been ground too coarsely. If the coffee is unacceptably bitter, perhaps the grind is too fine, with too-high levels of organic acids being extracted.
2. Temperature
Let’s hold all of our variables except temperature constant and see what happens. As with our coarseness experiment let’s consider the two ends of the extremes.
Temperature strongly influences solubility and rates of extraction. Yes, you can extract coffee with ice water. The three curves on our graph above get pulled to the right, so given enough time we can extract a decent cup of coffee. Cold brew coffee is made this way–ground beans are placed in cold water and allowed to “brew” in the fridge for up to a day.
The solubility of caffeine is moderately affected by temperature and the solubility of the organic acids is strongly affected by temperature. We would expect that a coffee brewed using this method would be lower in caffeine and much lower in bitterness than a coffee brewed using hot water.
Now, let’s extract our coffee using boiling water. The curves on our graph get scrunched up on the left-hand side. Everything gets extracted much quicker and the margin for error becomes much smaller if we try to limit bitter organic acid content.
Another complicating factor is that our volatile oils are just that–volatile. If we boil coffee, our flavor and aroma compounds get carried away in the steam. This can produce a coffee that is weak in taste, yet high in caffeine and organic acids.
3. Time
Let us keep our coarseness, temperature and water-to-coffee ratio variables constant and only consider the time variable. If we consider the ideal cup of coffee is one that has maximum caffeine and maximum volatile oils while limiting the bitter organic acids, we would consider 4 on our arbitrary timescale to be just about perfect.
If we only extract to 2 on the timescale we will have a coffee high in caffeine but weak, or underdeveloped, in flavor, aroma and bitterness. If we extract for too long, say to 8 on our timescale, our coffee will contain high amounts of organic acids, which can make it unacceptably bitter.
4. Coffee-to-water ratio
This brings us to our coffee-to-water ratio–perhaps the most subjective of all our tests. Too little coffee and even with all our variables optimized the coffee will taste weak. Too much coffee and the resulting brew will be too strong and overpowering.
This ratio depends on choice of extraction method:
· for a French press, or plunger, where the temperature of the water drops quickly, we need to have more coffee per unit of water
· if using a drip filter, the water temperature is higher than that in a plunger so a lower ratio is needed
· in modern espresso machines the volume of water can be changed to taste. Generally, the water temperature is maintained within the machine at around 97C (206 F). Too little water and the coffee is weak and underdeveloped; too much water and the coffee is bitter.
The generally accepted rule of thumb for the coffee-to-water ratio is approximately 10g (.35 oz.) of coffee to 200mL (.85 of a cup) of hot water. One heaping tablespoon is about 15g (.5 oz.) give or take a gram or two.
So there you have it. Optimize the coarseness of the grind, match this with the water temperature and the extraction time and make sure your coffee-to-water ratio is in the right ballpark. Or you can go down and visit your friendly local barista, have a chat, and let them do the thinking for you.
Don Brushett does not work for, consult to, own shares in or receive funding from any company or organization that would benefit from this article, and has no relevant affiliations. Southern Cross University does not contribute to the cost of running The Conversation. http://theconversation.com/who-funds-the-conversation-13921 The Conversation is funded by Howard Hughes Medical Institute, Robert Wood Johnson Foundation, Alfred P Sloan Foundation and William and Flora Hewlett Foundation. Our global publishing platform is funded by Commonwealth Bank of Australia.
By Don Brushett









