Techsomed’s thermal ablation therapy, a minimally invasive procedure for small tumors, has huge benefits over surgery or chemotherapy for patients and is a world first.
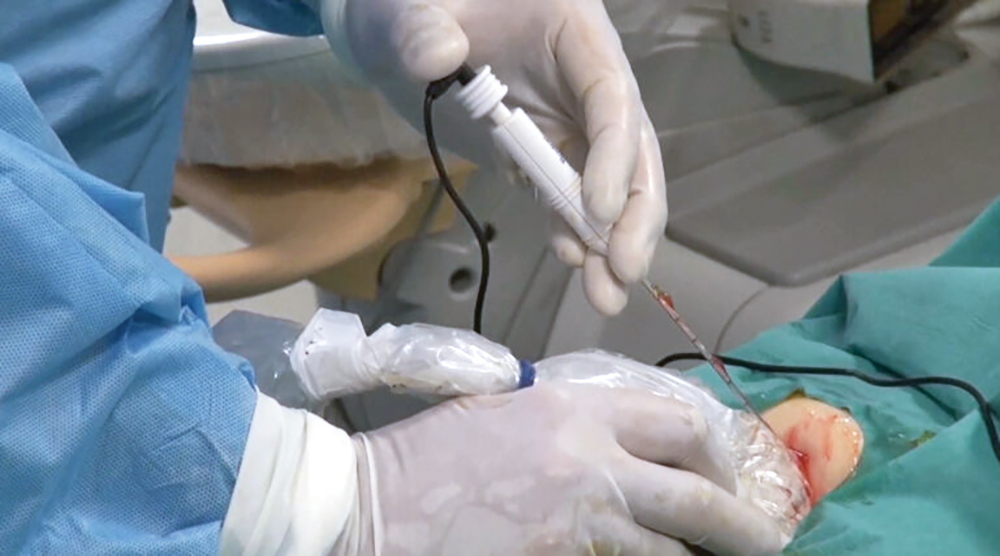
Hours in surgery, weeks in hospitals and months of recuperation–or a five-minute procedure under local anesthesia and straight back home with no side effects.
That’s the difference that AI (artificial intelligence) developed in Israel will now be making for patients diagnosed with liver cancer.
Techsomed, a medical imaging startup, allows physicians for the first time to see exactly what’s happening in real time while they destroy (or ablate) a tumor using targeted heat through a needle.
Thermal ablation therapy, a minimally invasive procedure for small tumors, has huge benefits over surgery or chemotherapy for the patients.
But it’s rarely used because of one significant drawback, namely that interventional radiologists, the people who perform the procedure, are effectively blindfolded.
They have ultrasound images to guide them, but their view of the tumor is obscured by a fog of tiny gas bubbles thrown up as they apply heat to the tissue.
So ablation currently relies largely on the physicians’ expertise and visual assessment. Without a clear picture, it’s hard for them to precisely target all of the tumor and none of the healthy tissue.
Techsomed reasoned that if life gave them lemons–or in this case tiny gas bubbles–they were going to make lemonade. They would turn the obstacle into the solution.
Making Sense of Bubbles
“This was the situation, and we decided to make the most of what we saw,” said CEO Yossi Abu, who founded the company in 2012.
He and his team, with backgrounds in defense, aviation, applied materials and semiconductors, were convinced that AI could make sense of the bubble chaos. They weren’t medical people, but they were problem solvers.
Their theory was that if they fed the AI enough ultrasound images, it could translate bubble activity into a clear picture of what was happening during the procedure.
And, critically, to predict what would happen over the next 24 hours, when the tumor continued responding to the aftereffects of the ablation.
It took seven years and $25 million. They adapted algorithms used to stabilize missiles, and technology from many other fields, to interpret ultrasound images.
“We trained an algorithm to correlate between this chaotic phenomenon called the ‘gas bubble effect’ and the damage to the tumor that will occur during the ablation procedure,” said Abu, a former engineer at Israel’s Rafael Advanced Defense Systems.
Game-Changing Technology
The AI developed by Techsomed, called BioTraceIO, shows the physician not only what’s happening in real time, but also how things will be the following day.
Ultrasound provides very chaotic images during the procedure, said Abu, but for generative AI it’s gold, allowing it to produce a live, high-quality ablation map.
This is game-changing technology, said Abu. It means ablation therapy, largely seen as a last resort, could now become the go-to treatment for liver tumors.
“We would like ablation treatment to become a first-line curative treatment. This is the mission. This is the vision,” said Abu.
“It’s impossible to grasp what the impact will be on patients. A patient undergoing surgery would need to be hospitalized for two weeks and their recovery takes months. It could cost $50,000 for the procedure and the risk to the patient is dramatic.
“Now you can replace that with a five-minute procedure under local anesthetic with no side effects. And the patient is released the same day.”
The Next Frontier
In time, he says, the company will go on to develop parallel technologies to guide physicians treating other solid tumors, such as the kidney and thyroid, as well as non-tumor ablation treatments, such as cardiac and chronic pain issues. Ultrasound can get to most organs except the brain.
They started with the liver because it’s a unique organ that can regenerate itself, so there was a bigger margin for error.
BioTraceIO was first used on a patient at Sheba Medical Center in Ramat Gan, central Israel, in 2016, and then at the University of Tokyo Hospital, Japan.
In January of this year, the US Food and Drug Administration (FDA) granted BioTraceIO De Novo clearance after tests on patients in several US hospitals.
De Novo (Latin for “from the beginning”) means it’s the only technology of its kind. In other words, a world first.
It will go live in US hospitals in the coming months.












